Water, the lifeblood of our planet, possesses a distinct freezing point that marks its transition into a solid state. Whether you’re an avid ice skater eager to comprehend the physics behind frosty rinks or a curious explorer seeking to unravel the secrets of nature’s icy wonders, understanding the freezing point of water is an intellectual pursuit that unlocks a realm of fascination.
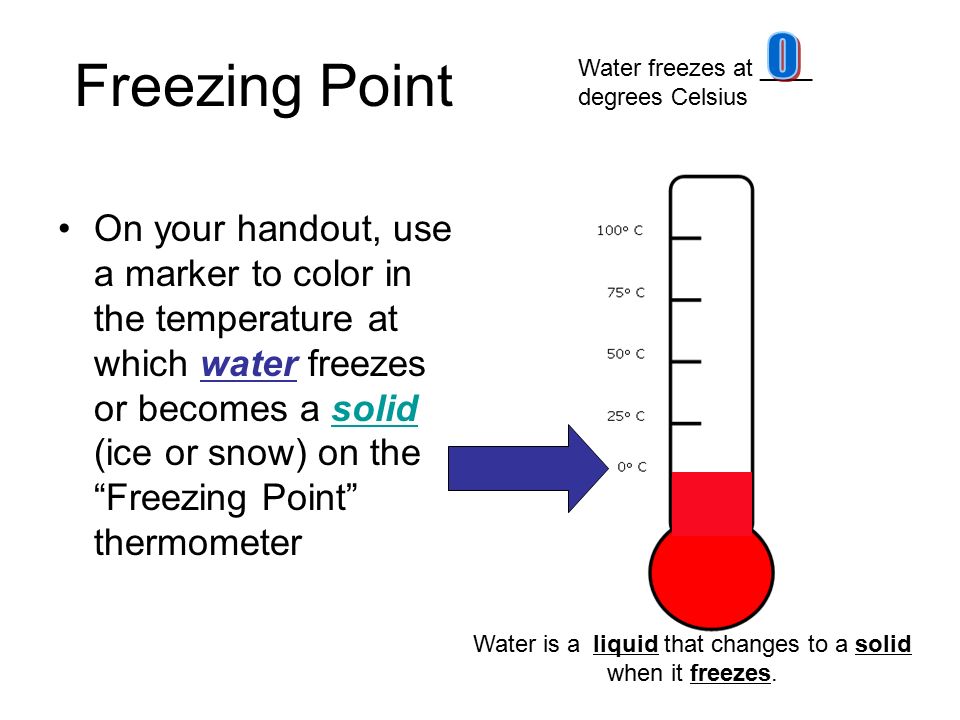
Image: www.micoope.com.gt
The Essence of the Freezing Point: A Story of Equilibrium
At its core, the freezing point of a substance represents the specific temperature at which it assumes a solid state. This phenomenon arises from a delicate equilibrium between two opposing forces: crystallization and melting. When the temperature drops below the freezing point, molecules within the liquid slow down and become more orderly, arranging themselves into rigid, ice-crystal structures. Simultaneously, the energy available for melting is reduced, causing water molecules to solidify rather than remain in a liquid form.
Decoding Degrees Fahrenheit: A Tale of Scientific Nomenclature
Fahrenheit, a scale named after the German physicist Daniel Gabriel Fahrenheit, denotes temperature values primarily used in the United States and a few other countries. It assigns 32°F as the freezing point of water, a convention widely adopted in everyday discourse. The scale, though not as predominant as Celsius globally, remains essential in certain fields like meteorology and forensic analysis.
The Extraordinary Physics of Ice Formation: A Marvel of Nature
The freezing of water unfolds as a captivating symphony of physical processes. In liquid water, molecules possess the freedom to roam about swiftly. As temperatures dip below the freezing point, these molecules experience a decrease in kinetic energy, resulting in slower motion and increased proximity to one another. This loss of molecular freedom facilitates the formation of stable hexagonal ice crystals, characterized by their unique six-sided symmetry.

Image: www.slideshare.net
Ecological Implications: The Unseen Impact of Water’s Freezing Point
The freezing point of water transcends scientific curiosity, delving into the realm of ecological significance. Landscapes across the globe bear living witness to its profound influence. When water bodies freeze, their surfaces become coated in a protective icy shield, safeguarding aquatic ecosystems from extreme cold. Furthermore, the formation of ice plays a pivotal role in moderating Earth’s climate by reflecting solar radiation back into space, contributing to the preservation of our planet’s delicate balance.
Engineering Marvels: Leveraging the Freezing Point of Water for Human Ingenuity
Human ingenuity has harnessed the freezing point of water for practical and innovative purposes. Ice skating, a globally beloved recreational activity, relies on the formation of ice surfaces at temperatures below freezing. The construction industry utilizes the freezing point to reinforce unstable ground during construction projects, injecting liquid nitrogen into the soil to create an ice-cemented foundation. Liquefying gases, a vital industrial process, hinges critically on achieving temperatures below the freezing point of water to transform gases into liquid form.
Navigating Temperature Extremes: The Human Body’s Response to Cold
The human body exhibits remarkable resilience in adapting to temperature changes, including the cold. Exposure to temperatures below freezing triggers a series of physiological responses to maintain optimal body temperature. Blood vessels near the body’s surface constrict, reducing heat loss, while shivering generates heat through involuntary muscle contractions. In extreme circumstances, prolonged exposure to sub-freezing temperatures can lead to hypothermia, a life-threatening condition.
Unveiling the Intriguing History: Ancient Observations and Scientific Milestones
Throughout history, humans have sought to understand the enigmatic properties of water, including its freezing point. Early civilizations witnessed and documented the transformative power of freezing, forming the basis for traditional knowledge systems. In the 17th century, Galileo Galilei invented the first thermometer, enabling the precise measurement of temperatures. Daniel Gabriel Fahrenheit, whose namesake scale we use today, later refined thermometer design, laying the groundwork for contemporary temperature measurement methodologies.
Ice in the Cosmos: Beyond Earth’s Aquatic Realms
Our exploration ventures into the cosmos reveal the incredible diversity of water’s existence. In the vast expanse of space, ice takes on new and awe-inspiring forms. Scientists have discovered the presence of water ice on countless celestial bodies, from the rings of Saturn to the moons of Jupiter and beyond. These cosmic icy wonders offer a glimpse into the universal prevalence of water and its potential role in the origins and evolution of life beyond Earth.
The Future of Water Research: Pushing the Boundaries of Knowledge
The pursuit of knowledge about water’s freezing point continues to drive scientific research. Advancements in technology have enabled sophisticated investigations into the behavior of water molecules at ultra-low temperatures. This ongoing research holds the promise of further revelations about the fundamental properties of water, with implications for diverse fields ranging from climate modeling to materials science.
What Is The Freezing Point Of Water In Degrees Fahrenheit
Conclusion
The exploration of the freezing point of water in degrees Fahrenheit unveils a world of scientific intrigue, practical applications, and environmental ramifications. Through its unique properties, water plays a profound role in our planet’s climate, enabling essential industrial processes, and serving as a playground for recreational activities. As research continues to probe deeper into the mysteries of water’s icy transformations, we stand in awe of its remarkable versatility and its indispensable role in our interconnected world.
