In the realm of chemistry, classifying substances as elements, compounds, or mixtures is fundamental to understanding their properties and behavior. But when it comes to the ubiquitous element aluminum, questions arise about its true chemical identity – is it an element, a compound, or a perplexing mixture? Join us on an enlightening journey as we dissect these concepts and reveal the true nature of aluminum.

Image: www.numerade.com
Delving into the Realm of Matter
To embark on this quest, let’s first establish our footing in the fundamental principles of chemistry. Different substances can be categorized into three distinct groups based on their constituent particles and how they interact: elements, compounds, and mixtures.
Elements are the building blocks of matter, indivisible substances comprising atoms of a single type. They possess unique properties, like density, melting point, and atomic number. Aluminum, with the symbol Al and atomic number 13, stands as a prime example of an element.
Compounds, on the other hand, are distinct from elements due to their composition of different atoms chemically bonded together in fixed proportions. These substances exhibit unique characteristics determined by the nature and ratio of their constituent atoms.
Lastly, mixtures represent a blend of two or more substances that may occur in various proportions. Unlike compounds, the components of a mixture retain their individual properties and may be easily separated by physical means like filtration, distillation, or chromatography.
Unveiling the Identity of Aluminum
With these concepts in mind, discerning the true nature of aluminum becomes apparent. As an element, aluminum exists in a singular atomic form, devoid of chemical combination with other elements. Its properties, such as low density, high electrical conductivity, and corrosion resistance, stem from the inherent nature of aluminum atoms themselves.
Aluminum, in its pure form, is extracted from raw materials like bauxite ore through various industrial processes, including refining and electrolysis. This single-element composition distinguishes aluminum from compounds and mixtures, firmly establishing it as an elemental substance.
Exploring the Diverse Applications of Aluminum
Understanding the elemental nature of aluminum unlocks a world of applications in various industries due to its unique properties. In construction, aluminum’s strength-to-weight ratio makes it an ideal material for building frames, roofing, and windows, offering durability and reduced energy consumption.
Its exceptional electrical conductivity has led to widespread use in electrical wiring, transformers, and capacitors. Additionally, aluminum finds extensive applications in transportation, food packaging, and even Aerospace, demonstrating its versatility and importance in modern society.
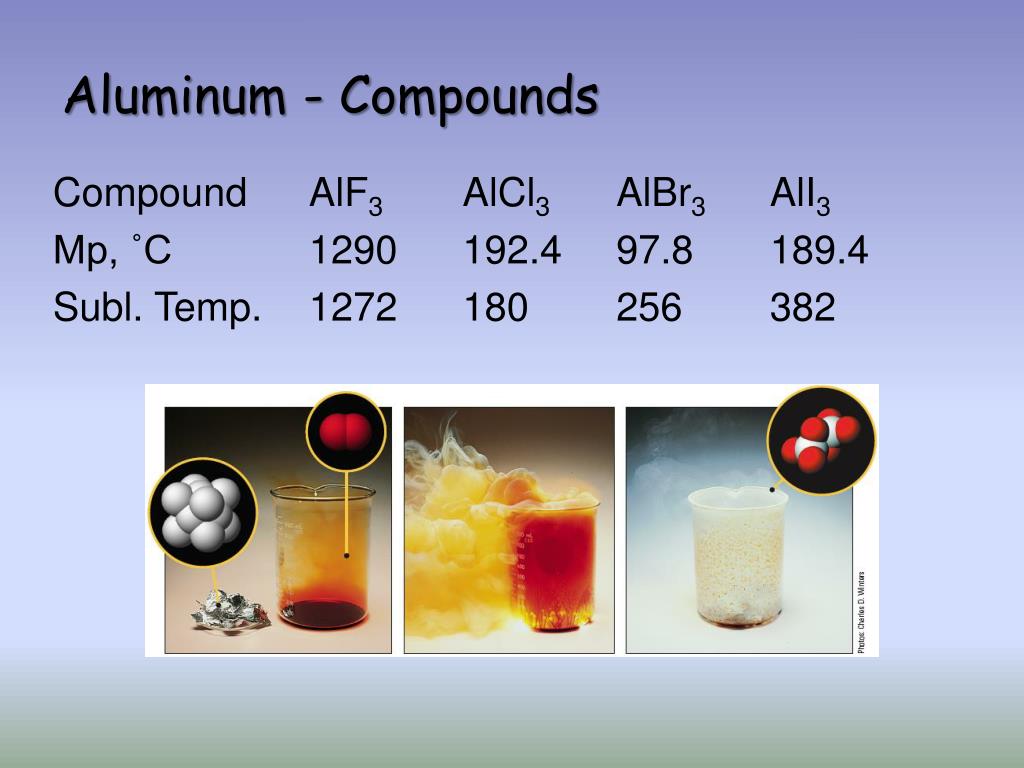
Image: www.slideserve.com
Is Aluminum An Element Compound Or Mixture
Achieving Clarity in Substance Classification
The classification of substances into elements, compounds, and mixtures forms the foundation of chemistry, enabling us to understand the diversity and behavior of matter that makes up our world. Aluminum, as an element, plays a crucial role in our daily lives and industries, due to its unique properties and versatile applications.
