Imagine this: you’re making your favorite dish, a delicious soup, and as you pour the stock into the pot, you notice something peculiar. Instead of smoothly mixing into a uniform broth, the stock seems to separate into tiny particles that float around stubbornly. This perplexing phenomenon is what we call a colloid—a fascinating mixture where one substance is dispersed as microscopic particles throughout another.
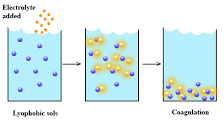
Image: thefactfactor.com
In the realm of colloids, there are two main categories: lyophilic and lyophobic. Lyophilic colloids, also known as hydrophilic colloids, have a special affinity for the liquid in which they are dispersed, resulting in a stable and homogeneous mixture. On the other hand, lyophobic colloids, or hydrophobic colloids, do not readily mix with the liquid and tend to form unstable dispersions. So, which of the following is a lyophilic colloid? Let’s explore the captivating world of colloids and uncover the answer.
Lyophilic Colloids: A Microscopic Symphony of Affinity
Lyophilic colloids are distinguished by their strong attraction to the liquid they reside in. This affinity, which is caused by the presence of polar groups or ions on the surface of the colloidal particles, promotes close association with the solvent molecules, creating a solvation layer around each particle. This solvation layer, acting as a protective shield, prevents the particles from aggregating or coagulating, ensuring the stability of the colloidal dispersion.
The realm of lyophilic colloids is vast and encompasses a diverse array of substances, including proteins, gelatin, starch, and gum arabic. These colloids find widespread applications in industries such as food, pharmaceuticals, and cosmetics, where their unique properties contribute to desirable traits like texture, stability, and efficacy.
Lyophobic Colloids: In Search of Stability in an Elusive Embrace
In contrast to their lyophilic counterparts, lyophobic colloids exhibit a natural aversion to the surrounding liquid. This aversion stems from the nonpolar nature of their surfaces, which inhibits solvation and leads to a lack of affinity with the solvent molecules. As a result, lyophobic colloids tend to form unstable dispersions that are prone to aggregation and coagulation.
However, lyophobic colloids can be coaxed into forming stable dispersions through the addition of stabilizing agents called emulsifiers. These emulsifiers, by reducing the surface tension between the colloidal particles and the liquid, promote particle dispersion and prevent them from clumping together. Lyophobic colloids find applications in industries such as paints, inks, and lubricants, where their unique properties contribute to desired characteristics such as flowability, color, and lubricity.
Examples and Applications: Where Lyophilic and Lyophobic Colloids Shine
To fully grasp the significance of lyophilic and lyophobic colloids, let’s delve into some real-world examples and applications:
-
Proteins: The primary components of our muscles and tissues, proteins, are nature’s lyophilic colloids. Their hydrophilic nature allows them to dissolve readily in water, forming stable solutions essential for biological processes like enzyme catalysis and nutrient transport.
-
Gelatin: Derived from collagen, gelatin is a lyophilic colloid widely used in the food industry. Its ability to form gels when heated and cooled is leveraged in the production of desserts, marshmallows, and photographic emulsions.
-
Colloidal Silver: A lyophobic colloid, colloidal silver is composed of tiny silver particles suspended in water. Despite its antimicrobial properties, colloidal silver can exhibit toxicity and is not approved for internal use. However, it finds application in wound dressings due to its ability to kill bacteria topically.
-
Paint: Lyophobic colloids play a pivotal role in the formulation of paints. Titanium dioxide, an inorganic lyophobic colloid, is widely used as a white pigment due to its ability to scatter light effectively. By controlling the size and shape of the titanium dioxide particles, manufacturers can tailor the opacity, brightness, and tint strength of paints.

Image: www.toppr.com
Which Of The Following Is A Lyophilic Colloid
Unveiling the Distinctive Nature of Colloids: Lyophilic versus Lyophobic
To summarize the key differences between lyophilic and lyophobic colloids:
-
Affinity for the Liquid: Lyophilic colloids have a strong affinity for the liquid, while lyophobic colloids have a weak or nonexistent affinity.
-
Stability: Lyophilic colloids form stable dispersions, while lyophobic colloids form unstable dispersions that tend to aggregate or coagulate.
-
Applications: Lyophilic colloids find applications in food, pharmaceuticals, and cosmetics, while lyophobic colloids find applications in paints, inks, and lubricants.
Understanding the lyophilic and lyophobic nature of colloids provides a deeper appreciation for the complexities of matter and its behavior at the nanoscale. These colloidal systems play a vital role in a wide spectrum of industries, shaping the products we use and enhancing our daily lives.
