Venturing into the Curious Case of Electron Affinity
Imagine yourself in a bustling marketplace, brimming with vendors offering wares fit for a chemist’s enchantment. Among their myriad bowls, each laden with a precious element, one particular commodity captures your attention—electrons. These elusive particles, eager to find a stable home, dance from atom to atom, orchestrating an intricate ballet known as chemical bonding. However, a curious pattern emerges amidst this chaotic waltz: some elements share an uncanny kinship, boasting an identical number of electrons in their outermost energy shell. This captivating phenomenon, where atoms shed their individuality and forge an unbreakable bond, is the essence of an isoelectronic series.
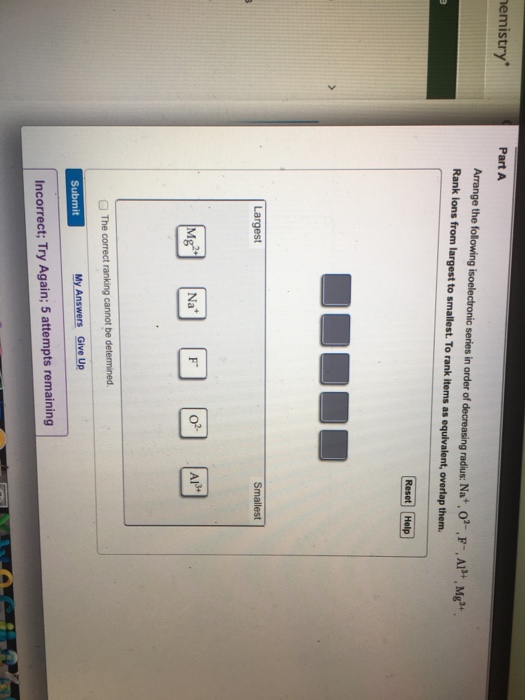
Image: www.chegg.com
Isoelectronic Series: A Concept Integral to Chemistry
An isoelectronic series, a vital concept in the tapestry of chemistry, encompasses a group of atoms or ions possessing the same electron configuration in their valence shell. This uniting characteristic profoundly impacts their chemical and physical properties, weaving together a web of shared traits and bestowing upon them a unique place in the periodic table.
History and Significance
The deep-rooted history of isoelectronic series dates back to the pioneering days of chemistry, when scientists began to unravel the secrets of the periodic arrangement of elements. As their understanding blossomed, they recognized the profound role of electron configuration, and with it, the significance of isoelectronic relationships emerged. Over the centuries, these series have become pivotal in explaining countless chemical phenomena, shaping the theoretical foundations of the field.
Defining Characteristics
Intrinsic to an isoelectronic series is the notion of a shared valence-electron count, tethering atoms or ions otherwise distinct in their identity. This electronic kinship extends beyond elemental species, encompassing ions as well, fostering unity amidst diverse chemical entities.
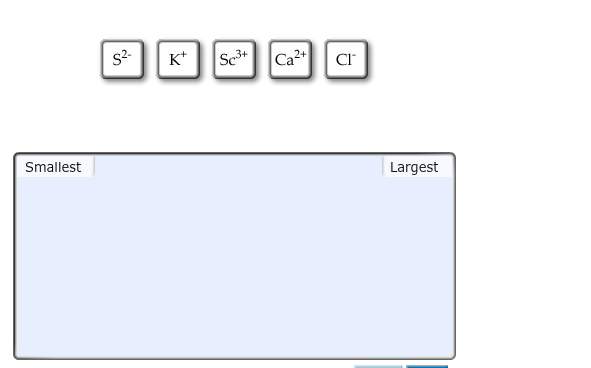
Image: www.chegg.com
Exploring the Wide-Ranging Applications of Isoelectronic Series
Spanning the diverse realms of chemistry, isoelectronic series play an indispensable role in unraveling the secrets of molecular behavior. From the intricate details of chemical bonding to the fascinating realm of atomic spectra, these series serve as an invaluable tool, paving the way for profound insights and groundbreaking discoveries.
Enlightening Chemical Bonding
Within the intricate dance of chemical bonding, isoelectronic series take center stage, illuminating the patterns and predictabilities of these fundamental interactions. By discerning the subtle interplay between electron configurations and bond strength, chemists gain invaluable knowledge, enabling them to design and manipulate materials with tailored properties.
Deciphering Atomic Spectra
Traveling beyond the realm of chemical bonding, isoelectronic series extend their influence to the vibrant world of atomic spectra. As atoms transition between energy levels, releasing photons of distinct wavelengths, the shared electron configuration within an isoelectronic series imprints its signature upon these emitted spectrums, providing a unique fingerprint for each element. These spectral patterns serve as powerful investigative tools, allowing scientists to probe the depths of atomic structure and unravel celestial mysteries.
Unveiling the Mendeleev Conundrum
In the enigmatic realm of the periodic table, isoelectronic series play a pivotal role in unriddling the puzzling inconsistencies of the Mendeleev ordering. Certain anomalies, once shrouded in mystery, are brought into sharp relief when viewed through the lens of isoelectronic relationships, revealing a hidden order amidst apparent chaos and providing a deeper understanding of the periodic system.
Delving into the Nuances of Isoelectronic Series
To embark on a deeper exploration of isoelectronic series, it is imperative to grasp a few key intricacies. The notion of electronegativity, a measure of an atom’s ability to attract electrons, proves pivotal in understanding the nuanced variations observed among these series. Elements within a given family, despite sharing the same number of valence electrons, may exhibit differences in electronegativity, leading to slight variations in chemical properties and unlocking new avenues for reactivity.
Furthermore, the concept of atomic radius, a measure of an atom’s size, deserves consideration. Within an isoelectronic series, atoms with a greater number of protons at their core boast a smaller atomic radius due to the increased electrostatic attraction between protons and electrons. This interplay between electronegativity and atomic radius introduces additional complexity, underscoring the influence of nuclear charge on the overall nature of an isoelectronic series.
Harnessing Isoelectronic Relationships: A Toolkit for Experiential Learning
Appropriating the wisdom gleaned from isoelectronic series, we can cultivate a deeper appreciation for the intricate choreography of electrons, unraveling the hidden connections between elements that breathe life into the tapestry of chemistry. Through a series of expertly crafted demonstrations, we invite you to immerse yourself in the transformative power of these relationships, gaining first-hand insights into their profound implications.
Experiment 1: Unmasking the Shared Reactivity of Isoelectronic Species
Gather a collection of potassium chloride and calcium fluoride, two ionic compounds that share an isoelectronic relationship via their chloride and fluoride ions. Armed with these chemical specimens, embark on a revealing experiment that unveils their remarkable resemblance in solubility and precipitation behavior. Stir each substance into water and observe the mesmerizing disappearance of both compounds into solution. Subsequently, introduce silver nitrate to the aqueous solutions and witness the telltale formation of silver chloride and silver fluoride precipitates, identical in their white, crystalline splendor. Through this hands-on experience, you will witness firsthand the shared chemical reactivity of isoelectronic species, solidifying your understanding of their kindred nature.
Experiment 2: Illuminating the Spectroscopic Fingerprint of Isoelectronic Series
Venture into the intriguing realm of spectroscopy and uncover the unique spectral signatures of isoelectronic species. Procure a hydrogen discharge tube and a helium discharge tube, both glowing with the radiant emission of excited atoms. Analyze the emitted light using a spectroscope and meticulously record the observed wavelengths. As you meticulously examine the resulting spectra, you will uncover striking similarities between the distinctive line patterns. The characteristic emission wavelengths, mirroring the shared electron configurations, will unveil the spectroscopic fingerprint that distinguishes each series, solidifying your appreciation for the profound influence of isoelectronic relationships on atomic spectra.
Frequently Asked Questions (FAQs): Unraveling the Mysteries of Isoelectronic Series
To cater to your inquisitive nature, we present a comprehensive FAQ section, meticulously crafted to address the most frequently encountered queries regarding isoelectronic series.
Question: What prominent examples of isoelectronic series can you provide?
Answer: Among the most well-known isoelectronic series are the alkali metals (Group 1) and the noble gases (Group 18), each characterized by their distinctive valence-electron configurations.
Question: How does electronegativity impact the properties within an isoelectronic series?
Answer: Electronegativity plays a pivotal role, subtly modulating the chemical properties of elements within a series. Despite sharing the same number of valence electrons, differences in electronegativity lead to variations in reactivity, forming the bedrock for their unique chemical personalities.
Which Of The Following Is An Isoelectronic Series
https://youtube.com/watch?v=znnHcFKMJ1A
Call to Action: Enriching Your Understanding of Chemistry
Dear readers, our journey into the captivating world of isoelectronic series has unveiled the profound impact of shared electron configurations on chemical and physical properties. May you be inspired to delve deeper into the realm of chemistry, exploring the periodic table with fresh eyes and a profound appreciation for the intricate dance of electrons. Share your thoughts and discoveries in the comments section below, fostering a vibrant exchange of ideas and enriching our collective understanding of this remarkable concept.
