Understanding Chemical Transformations
Chemical reactions are ubiquitous in our world, driving countless processes, from the tiniest biochemical reactions to the grandest celestial phenomena. These changes involve the rearrangement of atoms and molecules, forming new substances with distinct properties and characteristics. Distinguishing between chemical and physical changes is crucial for comprehending the nature of matter and the dynamics of our universe.
:max_bytes(150000):strip_icc()/TC_608334-chemical-change-examples-5aabebea31283400371a753e.png)
Image: www.thoughtco.com
Definition and Importance
A chemical change, also known as a chemical reaction, is a process that results in the formation of new substances with different compositions and properties. These changes involve the rearrangement of chemical bonds, forming new structural frameworks and altering the chemical makeup of the reactants. Chemical changes are irreversible, marking a permanent shift in the identity of the substances involved.
Understanding chemical reactions is essential for various reasons. They play a fundamental role in diverse scientific fields, including chemistry, biology, and materials science. Chemical reactions drive countless industrial processes, enabling the production of everything from pharmaceuticals to fertilizers to plastics. Moreover, chemical reactions are at the core of biological systems, governing our metabolic pathways, digestion, and energy production.
Determining Chemical Changes
Differentiating between chemical and physical changes is vital for grasping the fundamental nature of substances and their transformations. Physical changes, such as melting, boiling, or freezing, involve alterations in the physical state or form of the material, but they do not alter the chemical composition or structure. In contrast, chemical changes lead to the formation of new substances with distinct compositions and properties.
Observing changes in energy, the emission of gases or heat, and the occurrence of irreversible transformations can provide clues in identifying chemical reactions. However, to fully understand and predict the outcome of chemical changes, a deeper understanding of the principles behind these reactions is necessary.
Examples of Chemical Changes
Numerous everyday occurrences illustrate chemical changes. Rusting of iron, where iron reacts with oxygen to form iron oxide, is a classic example. Combustion, such as burning wood or fossil fuels, represents another type of chemical change, where substances react with oxygen to produce new compounds, releasing energy in the form of heat and light. Cooking is replete with chemical reactions, such as the Maillard reaction, responsible for the appetizing browning of food.
In photosynthesis, plants use sunlight to convert carbon dioxide and water into glucose, a fundamental energy source for life on Earth. Various digestive processes also involve chemical reactions, where enzymes break down complex carbohydrates and proteins into simpler molecules the body can utilize.
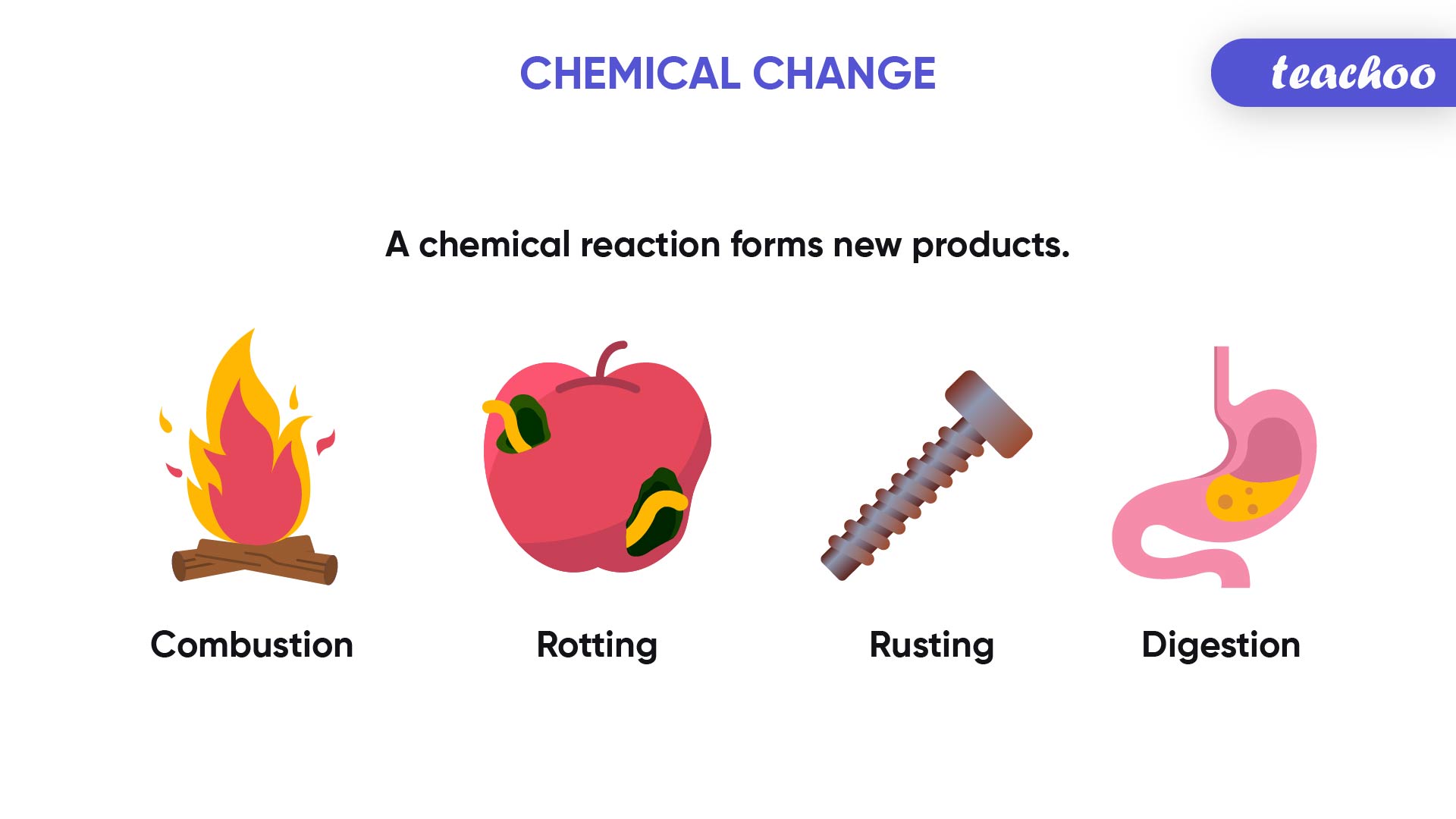
Image: www.teachoo.com
Distinguishing from Physical Changes
Understanding the key differences between chemical and physical changes aids inComprehending the spectrum of transformations matter can undergo. While physical changes involve alterations in physical properties such as shape, size, or color, they do not entail changes in chemical composition. Examples of physical changes include melting ice, stretching a rubber band, or crushing a can.
Applications and Impact
Chemical reactions are harnessed in countless industries and technologies, transforming raw materials into useful products. They form the cornerstone of pharmaceutical production, enabling the synthesis of life-saving medicines and treatments. In materials science, chemical reactions facilitate the development of advanced materials with tailored properties for various applications, such as in electronics, aerospace, and construction. Furthermore, studying chemical reactions underpins our understanding of environmental processes, from the impact of pollutants to the role of microorganisms in ecosystem dynamics.
Which Of The Following Is An Example Of Chemical Change
Conclusion
Chemical changes are fundamental processes that remodel the molecular architecture of substances, leading to the formation of new compounds with unique properties. Recognizing and understanding these transformations are essential for deciphering the workings of both natural and man-made systems. Chemical reactions drive countless industrial processes and underpin diverse scientific disciplines, shaping our technological advancements and deepening our comprehension of the universe.
