Unveiling the Aftermath of Energy Transformation
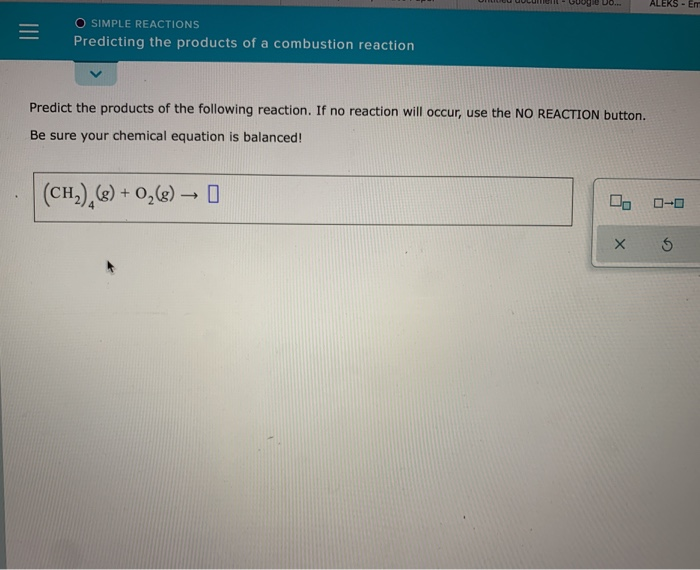
Image: www.chegg.com
Combustion reactions, characterized by their vibrant flames and release of energy, are ubiquitous in our daily lives, fueling everything from our cars to the cozy warmth of our fireplaces. But what exactly happens during these dynamic processes when fuel and oxygen dance together? What are the enigmatic products that arise from this fiery union? Embark on an illuminating journey to unravel the secrets of combustion reactions and uncover the transformative entities they unleash.
1. Carbon Dioxide: The Invisible Greenhouse
Carbon dioxide, an invisible yet potent greenhouse gas, is a central by-product of fuel-rich combustion. As carbon atoms gracefully combine with oxygen molecules, a transparent blanket of CO₂ envelops our atmosphere. While essential for plant growth, excessive carbon dioxide poses significant environmental concerns by contributing to global warming.
2. Water Vapor: An Atmospheric Transformer
Water vapor, the gaseous form of water, emerges as another pivotal product of combustion reactions. Hydrogen atoms, liberated from hydrocarbon fuels, form an intimate bond with oxygen, producing H2O molecules that ascend into the atmosphere. Water vapor serves both beneficial and adverse roles: nourishing clouds, yet influencing weather patterns and contributing to climate change.
3. Nitrogen Oxides: Invisible Pollutants
Nitrogen oxides, particularly nitrogen dioxide (NO2), are often unwelcome by-products of combustion. Nitrogen molecules, abundant in the air we breathe, react with oxygen under intense heat, forming these colorless yet hazardous compounds. Nitrogen oxides impact respiratory health, contribute to smog formation, and pose environmental risks.
4. Sulfur Dioxide: A Sour Scent
Sulfur dioxide, a pungent gas with a distinct odor, can accompany combustion reactions if sulfur is present in the fuel. Coal, for instance, releases sulfur dioxide into the atmosphere, which readily reacts with water, leading to acidic precipitation that jeopardizes aquatic ecosystems and damages infrastructure.
5. Particulate Matter: Tiny Air Pollutants
Particulate matter, an ensemble of microscopic airborne particles, emerges from incomplete combustion reactions. Soot, aerosols, and dust represent various forms of particulate matter. Their presence impairs air quality, aggravates respiratory illnesses, and adversely influences visibility.
6. Heat: A Smoldering Force
Heat stands as a critical product of combustion reactions. Fuel’s conversion into carbon dioxide, water vapor, and other compounds releases significant amounts of energy in the form of heat. This liberated energy finds applications in heating, power generation, and industrial processes.
Conclusion: Unveiling Combustion’s Mark
Combustion reactions unveil a captivating tapestry of products, each carrying its own significance. From the invisible greenhouse gas, carbon dioxide, to the hazardous pollutants, nitrogen oxides, each element reflects the transformative power inherent in this dynamic chemical process. Understanding these products empowers us to harness combustion’s energy effectively while mitigating its environmental impact, ensuring harmony between technological progress and the preservation of our planet.

Image: www.chemistrylearner.com
What Are The Products Of A Combustion Reaction
