Introduction:
In the intricate world of chemistry, covalent bonds play a pivotal role in forming and structuring molecules. These bonds, characterized by the sharing of electron pairs between atoms, are not always uniformly distributed and can possess slight differences in electrical charge. Understanding the factors that influence the polarity of covalent bonds offers invaluable insights into the properties and behavior of chemical compounds.
Factors Contributing to Bond Polarity:
Whether a covalent bond is likely to exhibit polarity or remain nonpolar largely depends on the electronegativity values of the atoms involved. Electronegativity measures an atom’s relative ability to attract shared electrons in a chemical bond. When atoms with varying electronegativities form a bond, a shift of electrons toward the more electronegative atom occurs, resulting in a polar bond. The more pronounced this electronegativity difference, the greater the polarity of the bond.
Electronegativity Differences:
The Pearson electronegativity scale provides a handy reference point for assessing the electronegativity values of different elements. Hydrogen, with an electronegativity of 2.2, serves as the benchmark for comparison. When bonding with an element more electronegative than itself (e.g., fluorine), the difference in electronegativity leads to significant polarity. In contrast, bonding between atoms with similar electronegativity values (e.g., carbon and hydrogen) results in nonpolar or weakly polar bonds.
Bond Distance and Bond Order:
In addition to electronegativity differences, the bond distance and bond order can influence bond polarity. Shorter bond distances, implying a reduced separation between the bonded atoms, tend to lead to more polar bonds. Similarly, higher bond orders, indicating multiple electron pairs shared between the atoms, generally result in enhanced bond polarity.
Orientation of Polar Bonds:
The orientation of polar bonds has profound implications for the overall molecular polarity. In molecules with two or more polar bonds, the molecular polarity depends on the vector sum of the individual bond dipoles. Dipole moments, representing the product of charge magnitude and distance, measure the polarity of bonds and molecules. The alignment of these dipoles determines the net polarity, with symmetric arrangements often canceling out and leading to nonpolar molecules, while asymmetric arrangements contribute to overall molecular polarity.
Consequences of Bond Polarity:
The polarity of covalent bonds affects their chemical behavior and influences intermolecular interactions. Polar molecules readily interact via dipole-dipole forces, aligning to minimize repulsive interactions and optimize attractive forces. These dipole-dipole interactions are crucial in determining many physical properties, such as melting point, boiling point, and solubility. Additionally, polar molecules exhibit polarizability, allowing them to deform and interact with electric fields.
Applications of Polarity in Chemistry:
The concept of bond polarity finds diverse applications in various chemical disciplines, including materials science, drug design, and biochemistry. Polar molecules possess unique properties that enable them to act as solvents, enhance reaction selectivity, and contribute to the structural integrity of materials. Their polar nature allows for selective interactions, facilitating targeted delivery and precise control in chemical processes.
Conclusion:
A fundamental understanding of polar covalent bonds in chemistry is essential in comprehending the behavior and properties of molecules and their intricate interactions in the world around us. The polarity of these bonds results from varying electronegativities, influences molecular polarity, and affects intermolecular forces. Recognizing how bond polarity arises and its significant implications gives chemists a powerful tool to design and manipulate materials at the molecular level. As chemistry progresses, further exploration of the fascinating consequences of bond polarity holds immense promise for shaping the future of scientific advancements.
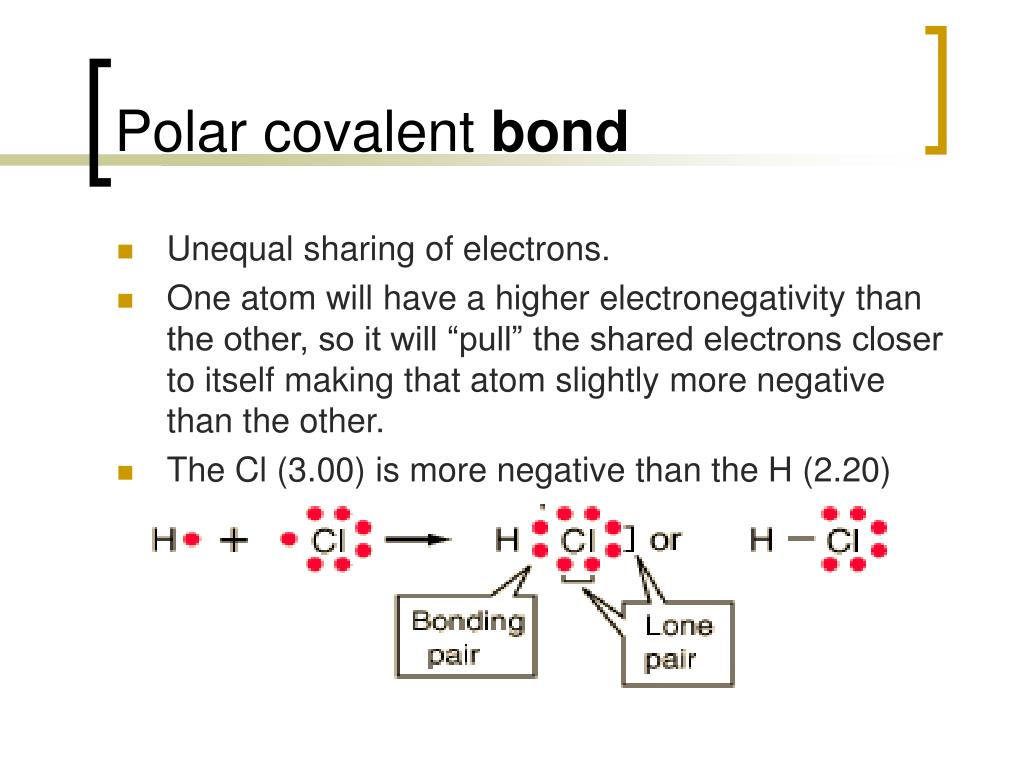
Image: www.slideserve.com

Image: www.chemistrylearner.com
A Covalent Bond Is Likely To Be Polar If
