Image: www.preclaboratories.com
In the realm of chemistry, the precise measurement of pH plays a pivotal role in understanding the myriad processes that shape our world. Whether it’s the acidity of a lemon, the purity of water, or the intricacies of bodily fluids, pH plays a vital role in understanding the dynamics of our surroundings. But what exactly is the pH of a neutral solution, and why is it so crucial? Join us as we delve into this intriguing scientific topic, uncovering the essential properties that define neutrality in chemical substances.
What is pH?
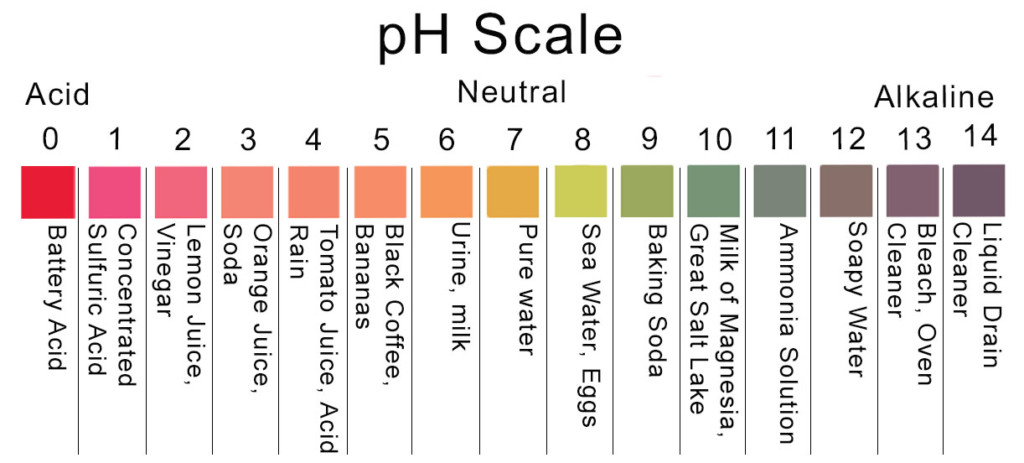
pH is a measure of how acidic or alkaline a solution is. It is expressed on a scale from 0 to 14, with 7 representing neutrality. Solutions with a pH below 7 are considered acidic, while those with a pH above 7 are considered alkaline or basic. Understanding pH is essential because it influences a wide range of chemical reactions and biological functions. For instance, human blood is slightly alkaline, with a pH of around 7.35, while seawater is slightly acidic, with a pH of approximately 8.1.
The Neutral Solution: A Precise Balance
By definition, a neutral solution has a pH of exactly 7. This represents the perfect equilibrium between acidity and alkalinity, where neither acid nor base dominates the solution. It is the ideal state for many biological systems and is found in pure water. Neutral solutions behave differently from acidic or alkaline solutions, ensuring stability and preventing harmful chemical reactions. For example, most enzymatic reactions in living organisms require a near-neutral pH to function optimally.
The Importance of Understanding Neutral Solutions
Understanding the principles of pH and neutral solutions is crucial not only for scientific research but also for various practical applications. In industries such as food manufacturing and pharmaceuticals, maintaining a neutral pH is vital for product quality and stability. In medicine, pH measurements help diagnose medical conditions and guide treatment decisions. Even in everyday life, understanding neutral solutions aids in selecting appropriate cleaning agents and optimizing the performance of household appliances.
How to Measure pH
Measuring pH can be done using various methods, both qualitatively and quantitatively. Litmus paper, a common qualitative indicator, changes color depending on the acidity or alkalinity of a solution. More precise measurements can be obtained using pH meters, electronic devices that provide accurate pH readings. These advanced tools are indispensable for both scientific research and industry settings.
Expert Insights: Unlocking the Realm of pH
Dr. Amelia Harper, a renowned chemist, elaborates on the significance of neutral solutions in the field: “Neutral solutions represent a delicate balance that underpins the very fabric of chemical processes. They create an optimal environment for countless reactions, facilitate biological functions, and are essential for the stability of vital substances.”
Conclusion
The pH of a neutral solution, with its precise value of 7, stands as a testament to the harmony that can exist between acidity and alkalinity. By uncovering the mysteries of pH, we gain a deeper understanding of the world around us and can harness its power to improve countless aspects of our lives. As we continue to explore this fascinating realm of chemistry, let us always seek the true and unbiased information that empowers us with knowledge and guides our scientific endeavors.

Image: www.youtube.com
What Is The Ph Of Neutral Solution
https://youtube.com/watch?v=YS2mbXGpqr8
