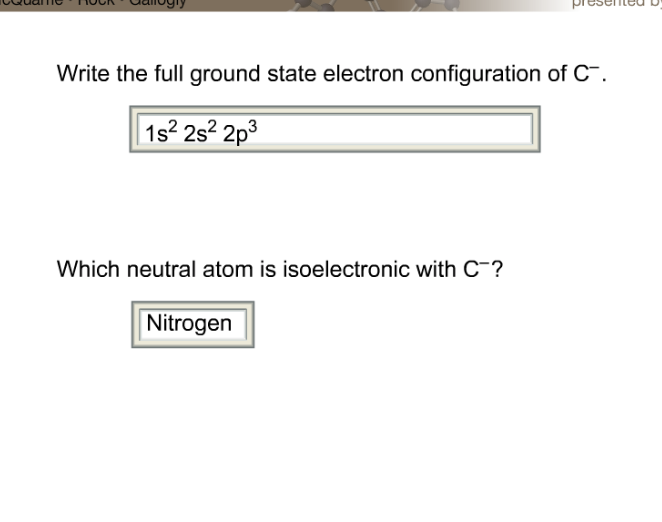
Image: www.chegg.com
In the vast expanse of the atomic realm, our fascination with the intricate tapestry woven by electrons has been an endless pursuit. Among the elements that grace the periodic table, silver (Ag) stands out as a shimmering testament to the exquisite dance of subatomic particles. To fully understand the remarkable properties of this precious metal, we must embark on a voyage into the depths of its electron configuration.
The electron configuration of an atom describes the arrangement of electrons within its energy levels and orbitals. It holds the key to understanding the chemical and physical characteristics of an element and serves as a blueprint for the molecular interactions that shape our world.
Unveiling the Electron Configuration of Silver
The ground-state electron configuration of silver, denoted as Ag, is:
[Kr] 4d10 5s1
This shorthand notation represents a layered structure of atomic orbitals, where electrons occupy specific energy levels defined by their principal energy level (n) and their angular momentum.
-
The first term, [Kr], refers to the electron configuration of krypton (atomic number 36). This indicates that the first 36 electrons of silver have the same electron configuration as the noble gas krypton. This “core” configuration provides a stable and chemically inert foundation.
-
The remaining 19 electrons of silver are distributed among the 4d and 5s orbitals:
-
4d10 represents the 10 electrons that occupy five 4d orbitals with two electrons each.
-
5s1 represents the remaining single electron that inhabits the solitary 5s orbital.
This configuration reveals that silver has an outermost electron in the 5s orbital, which is the valence electron responsible for its chemical reactivity.
The Significance of Electron Configuration
Understanding the electron configuration of silver unlocks a wealth of insights into its properties and behavior.
-
Chemical Reactivity: The presence of a single valence electron makes silver a relatively reactive element. This characteristic allows it to form bonds with other atoms readily, explaining its use in a wide range of chemical reactions.
-
Electrical Conductivity: The availability of the valence electron gives silver excellent electrical conductivity. This property makes it an ideal material for electrical wires, connectors, and other electronic components.
-
Reflectivity: The outermost 5s electron can absorb and reflect energy, providing silver with its characteristic lustrous and reflective appearance. This property has made it a popular choice in jewelry and decorative applications.
-
Antibacterial Properties: Silver ions, which can form when silver atoms lose their valence electron, have been shown to possess antibacterial properties. This effect has led to the use of silver in medical instruments, wound care products, and consumer goods.
Conclusion
The electron configuration of silver serves as a concise yet profound description of this versatile element’s atomic structure. Comprehending this configuration not only provides a fascinating glimpse into the intricate dance of subatomic particles but also unveils the scientific underpinnings of silver’s extraordinary properties and practical applications. As we delve deeper into the world of atoms and their electronic configurations, we unlock a treasure trove of knowledge that continues to shape and inspire our understanding of the physical world around us.
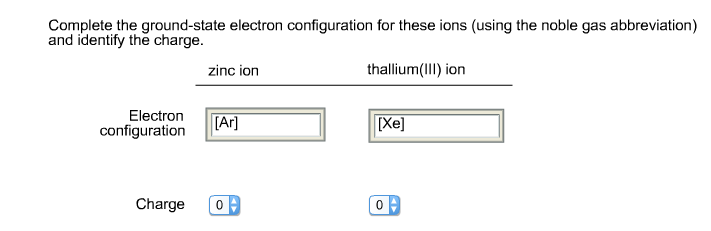
Image: www.chegg.com
Write The Complete Ground-State Electron Configuration Of Ag.
